Background: There is limited data on the real-world application of patient-reported outcomes (PROs) and their clinical utility in patients with acute leukemias or myelodysplastic syndrome (MDS). While the associations of PROs with clinical outcomes, such as overall survival (OS) and acute care utilization have been established in patients with solid tumors (Basch E et al. JAMA, 2017.318(2):197-198), these same associations are not well known in patients with hematologic malignancies.
Objectives: We assessed the characteristics of baseline PRO measurements in patients with newly diagnosed acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL) or myelodysplastic syndrome (MDS) and examined their associations with OS as well as time to acute care use (TAC).
Methods: This retrospective cohort study was conducted on patients with newly diagnosed AML, ALL or MDS between 11/2018-02/2022 who were receiving care at our institution and completed a baseline PRO survey within 30 days of therapy initiation. The PRO survey consisted of a 14-question assessment from the National Cancer Institute's PRO Version of The Common Terminology Criteria for Adverse Events. Each of the symptom domains of the survey are measured on a 1-5 Likert scale. OS was defined as time from therapy initiation to the time of death. TAC was defined as time from therapy initiation to first unplanned acute care visit (emergency room visit or unplanned admission). OS curves were estimated using the Kaplan-Meier method and compared using log-rank tests. Cox proportional-hazards regression modeling was used to compare the hazards of death and acute care use between groups.
Results: 56 patients were included with a median age of 63 years (range 26-79). The cohort consisted of more males, non-Hispanic Whites, and patients that received intensive initial therapy (Table 1). Fatigue was the most frequently reported symptom (n=49, 87.5%, mean score of 2.90/5 (SD 1.30), with a majority reporting mild or no symptoms (score < 4, n=33, 58.9%) and 28.6% (n=16) reporting more severe symptoms (scores > or = 4). Similarly, most patients in this cohort reported mild or no symptoms in terms of anorexia, anxiety, or sadness (Table 1).
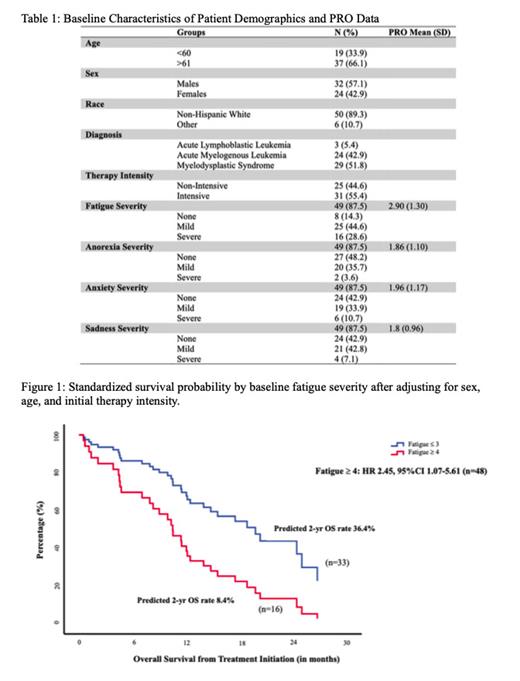
In terms of OS, univariate models for the presence of fatigue, anorexia, anxiety, or sadness was not associated with significantly higher hazard of death. On multivariate analyses adjusting for age, sex, and treatment intensity, every point increase in reported fatigue severity was associated with a 41% increased hazard of death (HR 1.41,95% CI: 1.03-1.93). When stratified by fatigue severity (> or = 4 severe vs < or = 3 mild/no symptoms), patients reported severe fatigue had an 145% increased hazard of death compared to those reported mild/no fatigue (HR 2.45 (95% CI: 1.07-5.61) (Figure 1). Multivariate analyses for anorexia, anxiety, and sadness were not associated with significant differences after adjusting for the above covariates, respectively.
Fourteen (25.0%) and 24 (42.9%) patients in this cohort had at least one unplanned acute care visit within 30 and 90 days from initiation of therapy, respectively. The median TAC for this cohort was 4.15 months (range 0.60-27.20 months). Univariate regression modeling revealed no differences for any of the PROs examined in this study. Similarly, no differences were observed in multivariate analyses for each of the PROs after adjusting for age, sex, and therapy intensity.
Conclusions: Fatigue was the most severe and frequently reported baseline symptom among patients with newly diagnosed acute leukemia or MDS. Patients reporting increased fatigue were found to have a higher hazard of death compared to those who did not after adjusting for age, sex, and treatment intensity. The characteristics and PROs examined in this study were not associated with TAC or predictive of acute care use at different time points from therapy initiation. Future work is needed to understand how PRO data can be used to identify higher-risk patients prior to treatment initiation.
Disclosures
Perl:BMS: Honoraria; Immunogen: Honoraria; Genentech: Honoraria; BerGen Bio: Honoraria; Beat AML: Other: Participation on a Data Safety Monitoring Board or Advisory Board; Foghorn: Consultancy; Forma: Consultancy; Syndax: Research Funding; FujiFilm: Research Funding; Bayer: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi-Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Aptose: Honoraria; Rigel: Honoraria; Actinium: Honoraria. Luger:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria; Onconova: Research Funding; Novartis: Consultancy; AbbVie: Membership on an entity's Board of Directors or advisory committees; Marker Therapeutics: Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria. Frey:Sana Biotechnology: Consultancy; Kite Pharma: Consultancy. Porter:Wiley and Sons Publishing: Honoraria; Sana Therapeutics: Consultancy, Current equity holder in publicly-traded company; Tmunity: Patents & Royalties; Novartis: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Mirror Biologics: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Genentech: Current equity holder in publicly-traded company; DeCart: Membership on an entity's Board of Directors or advisory committees; Capstan Bio: Honoraria; BMS: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Angiocrine Bio: Membership on an entity's Board of Directors or advisory committees. Pratz:Roche: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Jazz Pharamceuticals: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Research Funding; AbbVie: Consultancy, Research Funding. Lai:Jazz: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy; Daiichi: Consultancy; Taiho: Consultancy; Genentech: Consultancy; BMS: Consultancy; Rigel: Consultancy; Astellas: Consultancy, Speakers Bureau; Pfizer: Consultancy; AbbVie: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal